X-ray fluorescence (XRF) is a non-destructive analytical method used to determine elemental concentrations in various materials.
XRF works by striking a sample with an x-ray beam from an x-ray tube, causing characteristic x-rays to fluoresce from each element in the sample. A detector measures the energy and intensity (number of x-rays per second at a specific energy) of each X-ray, which is transformed into an elemental concentration using either a non-standard technique such as fundamental parameters or user-generated calibration curves.
The presence of an element is identified by the element’s characteristic X-ray emission wavelength or energy. The amount of an element present is quantified by measuring the intensity of that element’s characteristic X-ray emission.
 1- All atoms have a fixed number of electrons. These electrons are arranged in orbitals around the nucleus. Energy Dispersive XRF (EDXRF) typically captures activity in the first three electron orbitals, the K, L, and M lines.
1- All atoms have a fixed number of electrons. These electrons are arranged in orbitals around the nucleus. Energy Dispersive XRF (EDXRF) typically captures activity in the first three electron orbitals, the K, L, and M lines.
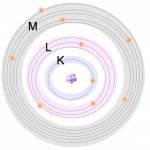 2- These electrons are arranged in orbitals around the nucleus. Energy Dispersive XRF (EDXRF) typically captures activity in the first three electron orbitals, the K, L, and M lines.
2- These electrons are arranged in orbitals around the nucleus. Energy Dispersive XRF (EDXRF) typically captures activity in the first three electron orbitals, the K, L, and M lines.
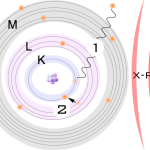 3- The primary photons from the X-ray tube have high enough energy that it knocks electrons out of the innermost orbitals, creating a vacancy (1). An electron from an outer orbital will move into the newly vacant space at the inner orbital to regain stability within the atom (2).
3- The primary photons from the X-ray tube have high enough energy that it knocks electrons out of the innermost orbitals, creating a vacancy (1). An electron from an outer orbital will move into the newly vacant space at the inner orbital to regain stability within the atom (2).
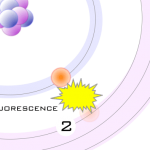 4- As the electron from the outer orbital moves into the inner orbital, it releases energy in the form of a secondary X-ray photon. This energy release is known as fluorescence. All elements produce fluorescence “characteristic” to themselves. Each element’s fluorescence is unique to itself.
4- As the electron from the outer orbital moves into the inner orbital, it releases energy in the form of a secondary X-ray photon. This energy release is known as fluorescence. All elements produce fluorescence “characteristic” to themselves. Each element’s fluorescence is unique to itself.
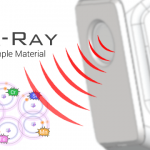 5- High-energy primary X-ray photons are emitted from an X-ray tube and strike the sample
5- High-energy primary X-ray photons are emitted from an X-ray tube and strike the sample
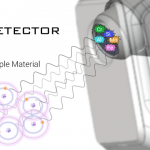 6- The fluorescent energy is transferred to a detector, where it is absorbed and transferred into an electrical signal and then into a number (digitized).
6- The fluorescent energy is transferred to a detector, where it is absorbed and transferred into an electrical signal and then into a number (digitized).

Results can be viewed in the form of percentages, or as spectrum. The XRF will process (digitize, count) about 200,000 or more x-rays every second. These detected x-rays form a spectrum. Each peak in the spectrum is from a characteristic x-ray that was emitted by a specific element, like Cr, or Ni, etc.
The height of the peak is proportional to concentration of the element. The peak height is converted to a percentage or ppm of that element via a calibration method – either fundamental parameters or factory or user-derived empirical calibrations (see below).