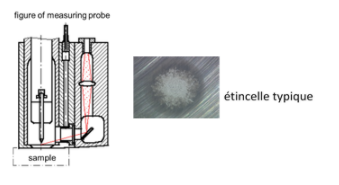
Optical Emission Spectrometry (OES) consists of applying electrical energy in the form of an arc (in the air) or a spark (under argon) generated between an electrode and a metal sample. the vaporized atoms are then brought to a state of high energy in a “discharge plasma”.
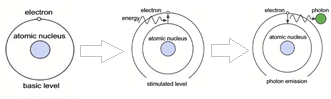
These atoms and ions excited in the discharge plasma create an emission spectrum specific to each element. Thus, a single element generates many characteristic emission spectral lines.
The wavelengths emitted are characteristic of the chemical element
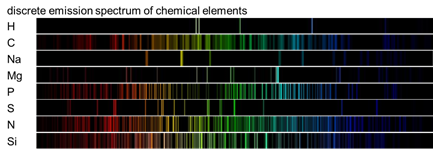
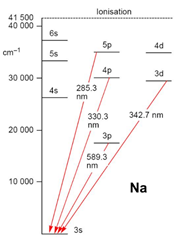
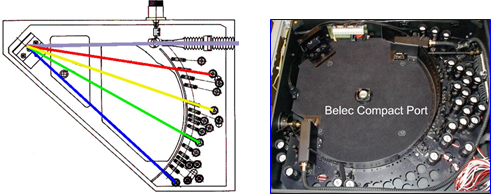
Therefore, it can be said that the light generated by the discharge is a collection of the spectral lines generated by the elements present in the sample.
This light is divided by a diffraction grating to extract the emission spectrum of the elements. The intensity of each emission spectrum depends on the concentration of the element in the sample. The detectors (photomultiplier tubes and / or CCD sensors) measure the intensity of the spectrum of each element in order to perform a quantitative analysis of these elements.
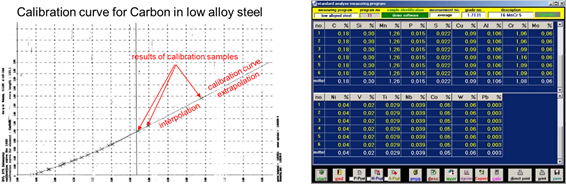
Calculation of quantitative results:
The calculation of the concentration of each element is based on comparing the results with a set of previously measured calibration samples.
Each element has many calibration curves specific to the matrix (base Fe, Cu, Al, …) and the measured concentration.